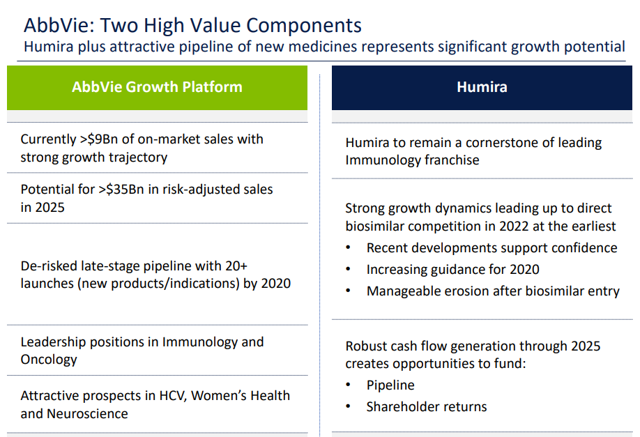
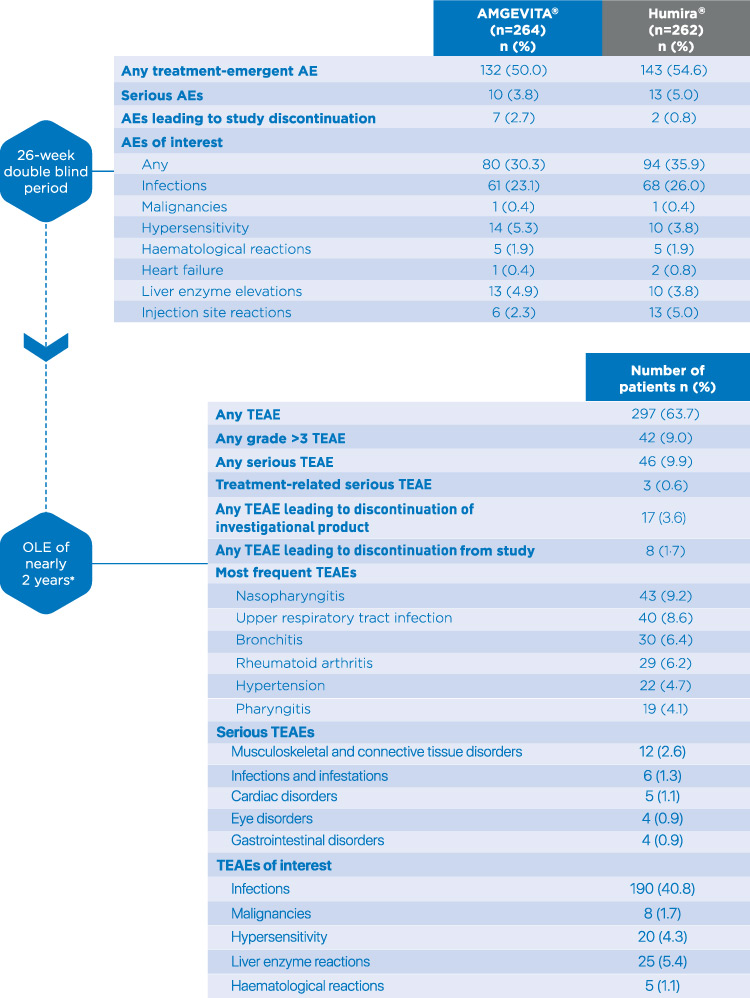
These highlights do not include all the information needed to use IMRALDI safely and effectively. See full prescribing information for IMRALDI.IMRALDI (adalimumab-xxxx) injection, for subcutaneous useInitial U.S. Approval: YYYY IMRALDI (adalimumab-xxxx) is

These highlights do not include all the information needed to use IMRALDI safely and effectively. See full prescribing information for IMRALDI.IMRALDI (adalimumab-xxxx) injection, for subcutaneous useInitial U.S. Approval: YYYY IMRALDI (adalimumab-xxxx) is