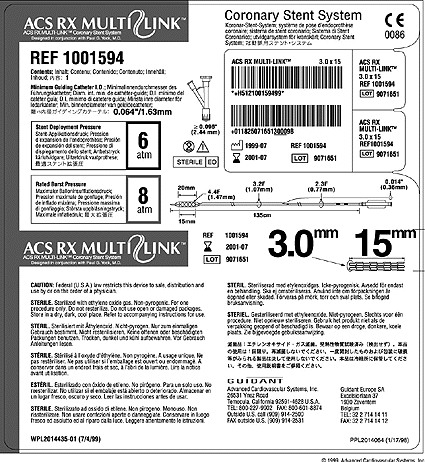
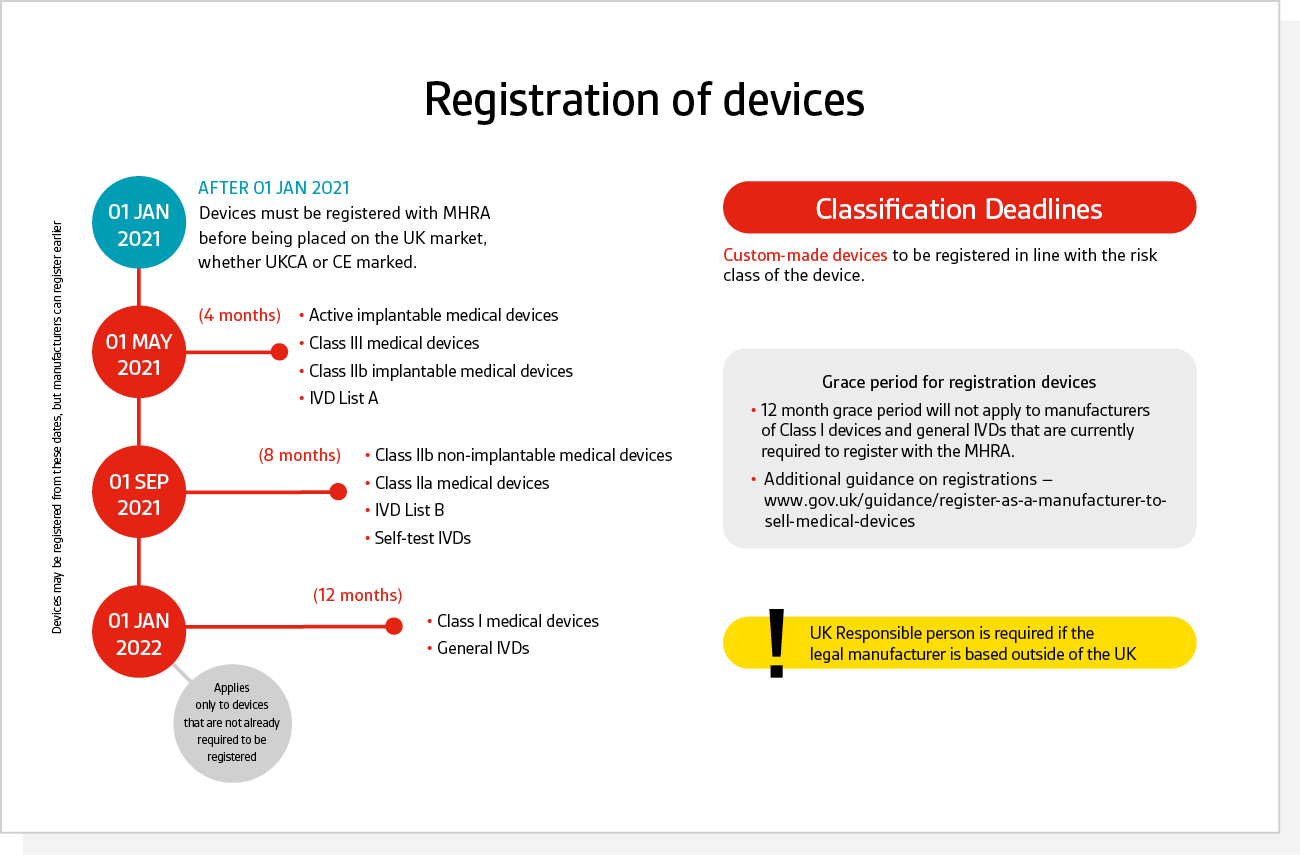
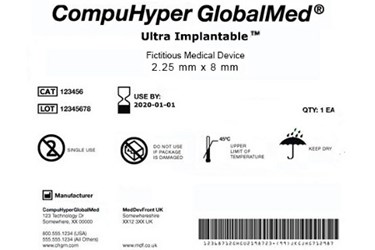
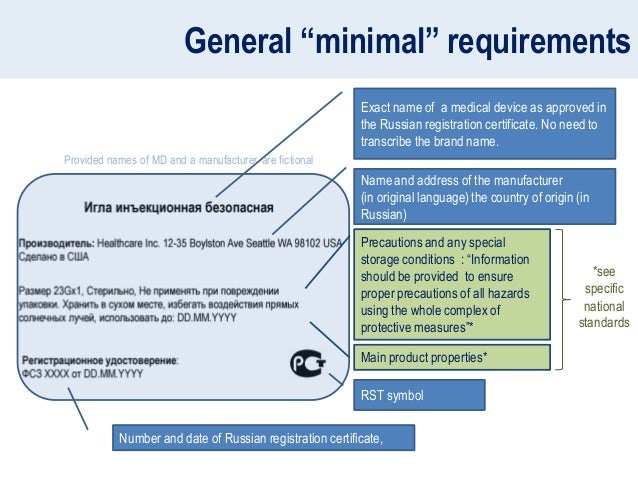
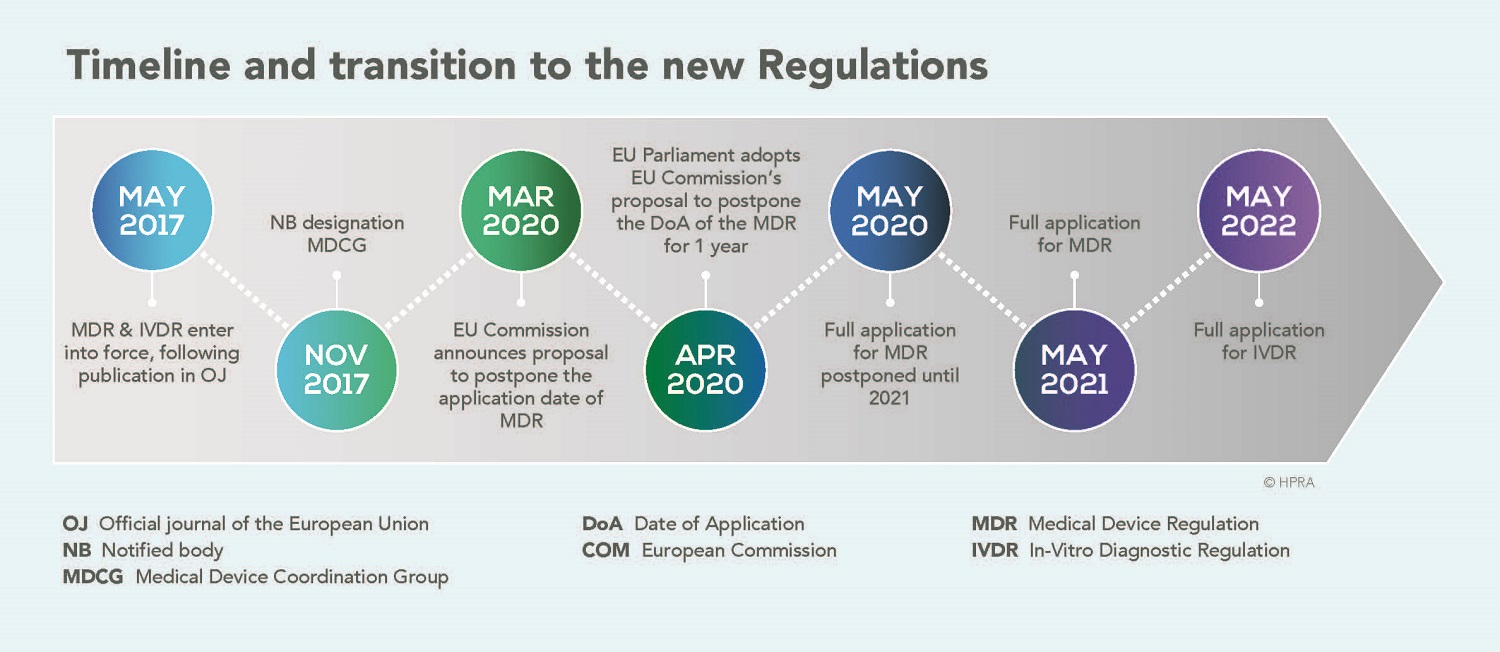
EN ISO 15223-1:2016/prA1 - Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements (ISO 15223-1:2016)
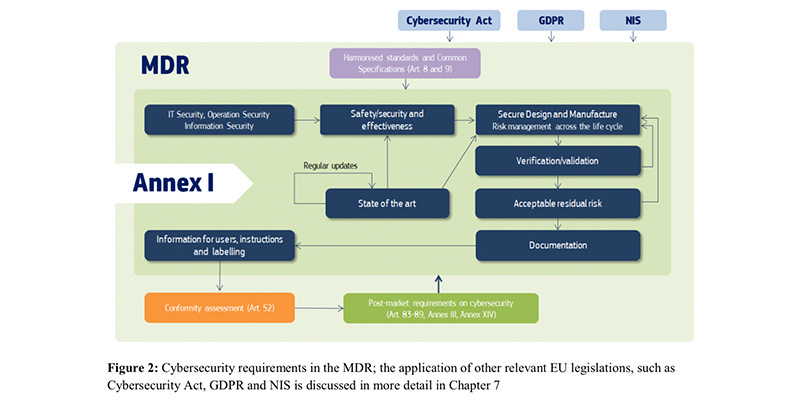
Medical Device Regulation: EU to give €100bn MedTech industry a security health check | The Daily Swig

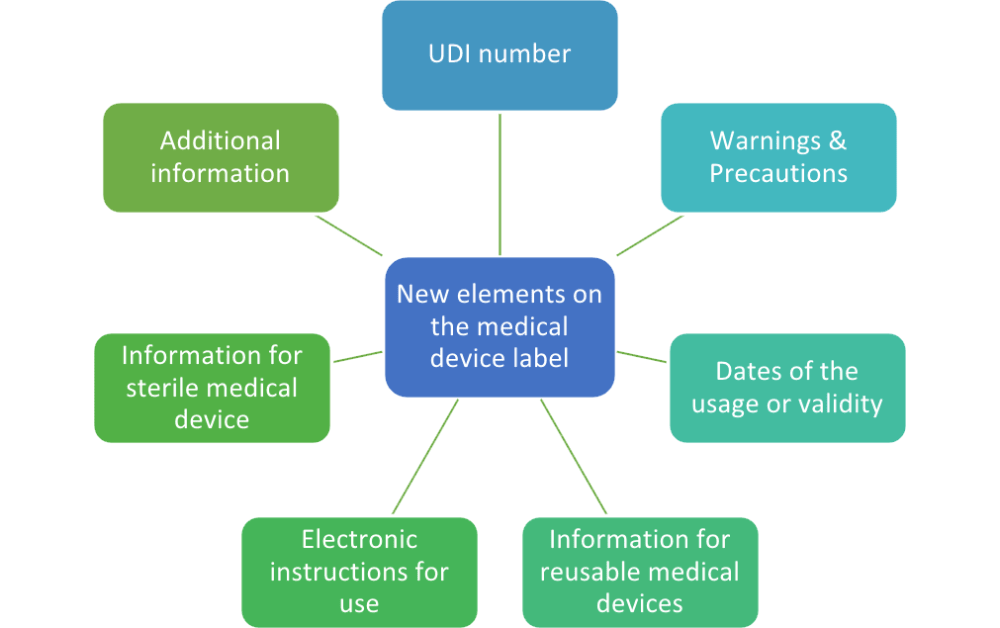
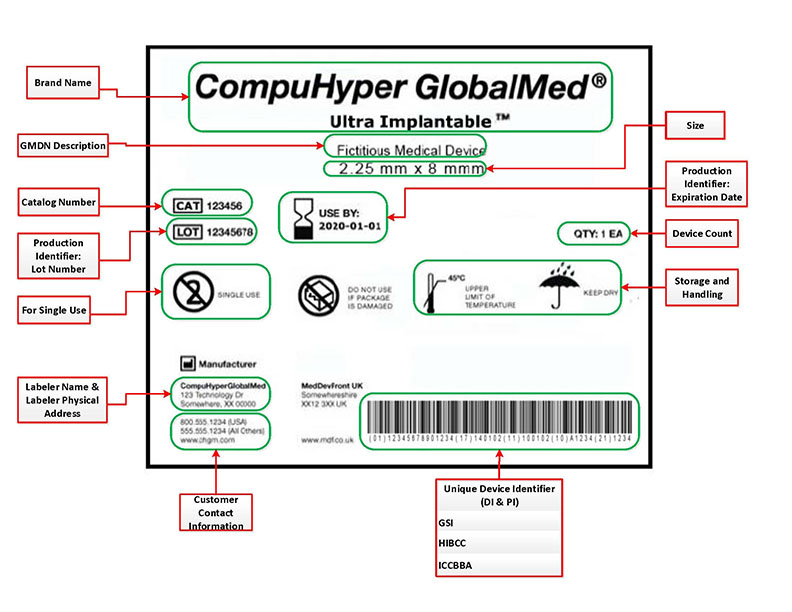
EU MDR - Medical Device Labeling Changes & Challenges - Regulatory, Clinical Consulting Services to Biopharma & Medical Device Companies