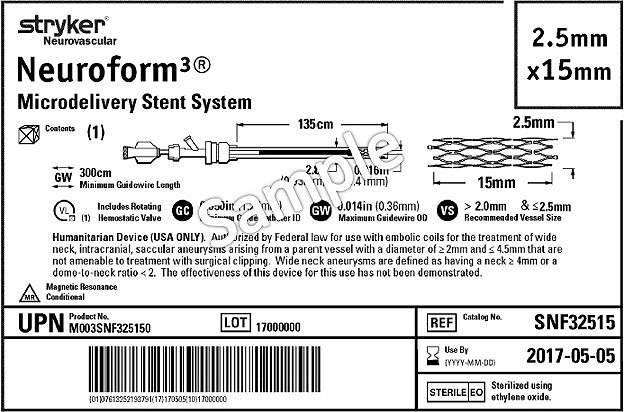
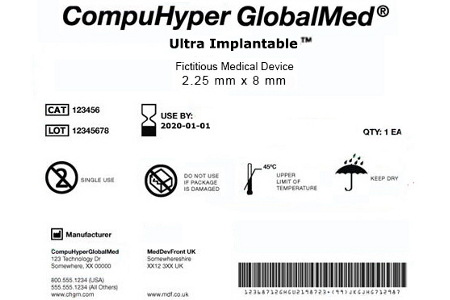
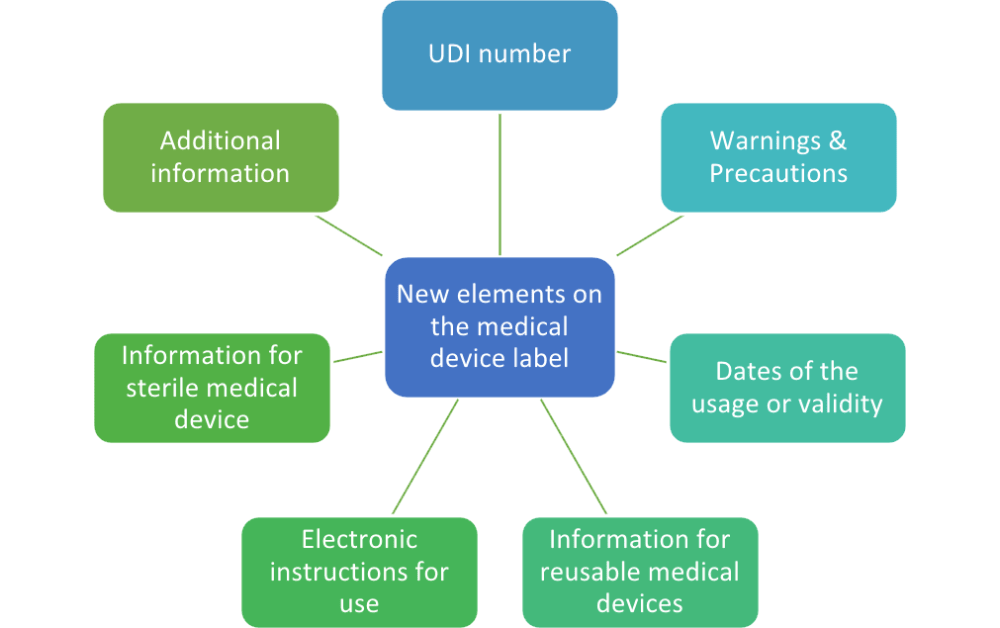
Health Canada's Action Plan on Medical Devices: Continuously Improving Safety, Effectiveness and Quality - Canada.ca
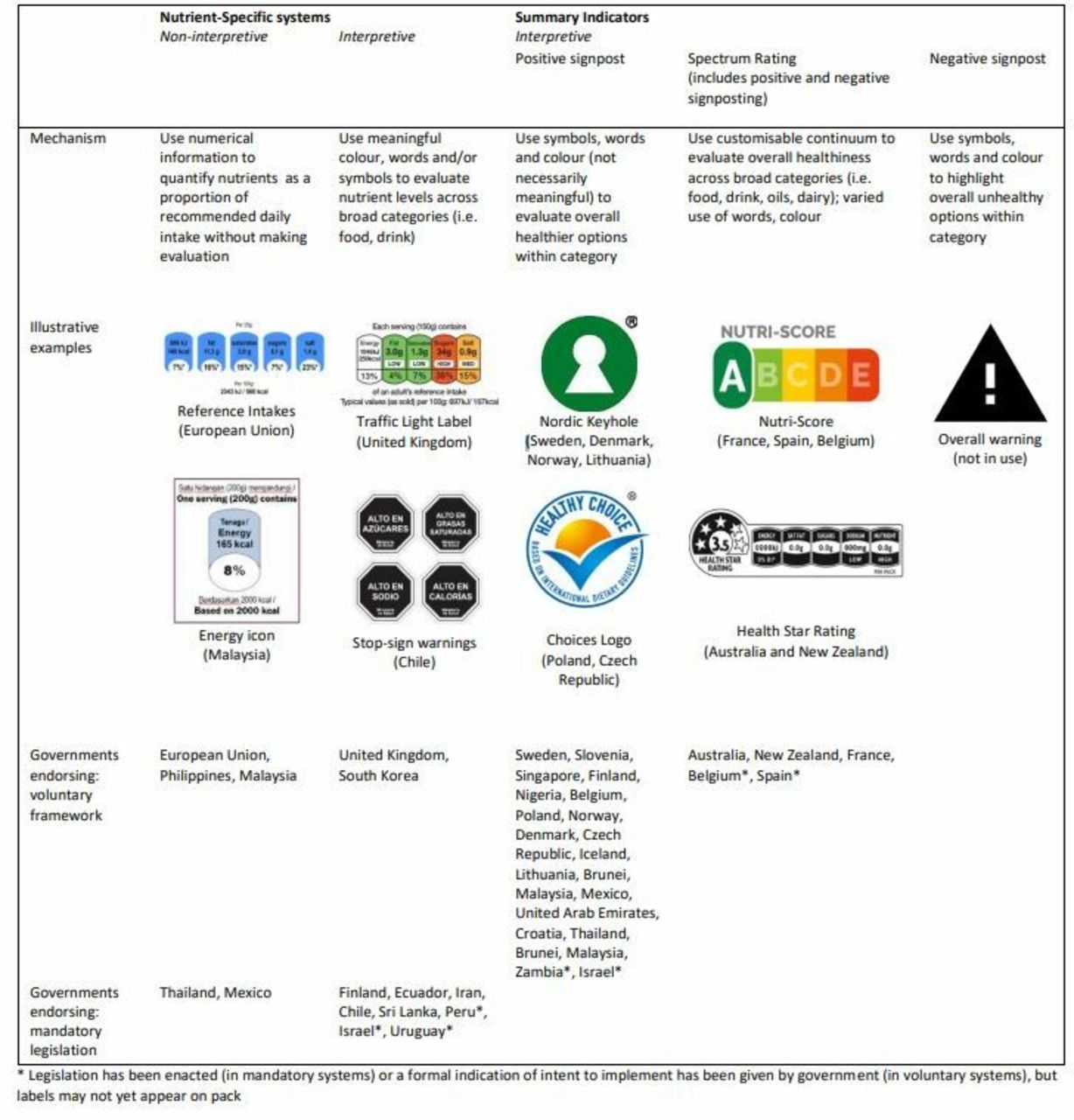
Front-of-pack nutrition labelling to promote healthier diets: current practice and opportunities to strengthen regulation worldwide | BMJ Global Health