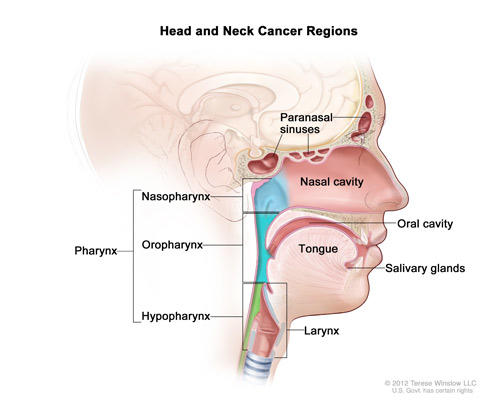
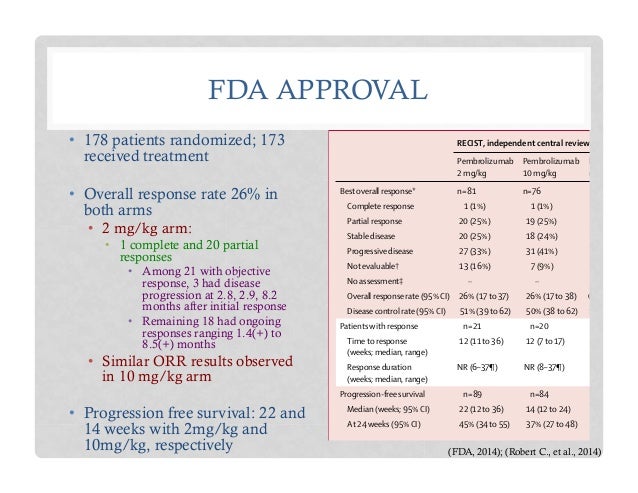
FDA Approves KEYTRUDA® (pembrolizumab) for the Treatment of Patients with Metastatic Non-Small Cell Lung Cancer Whose Tumors Express PD-L1 with Disease Progression On or After Platinum-Containing Chemotherapy - Merck.com
![PDF] FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar PDF] FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d87ced43628213babff1c81f9a45fcf02a22d940/2-Figure1-1.png)
PDF] FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar

A reality check of the accelerated approval of immune-checkpoint inhibitors | Nature Reviews Clinical Oncology

Pembrolizumab FDA approved for adjuvant treatment of melanoma – Medical Writer Agency | 醫學作家香港 | 醫學寫作 | MediPR | MediPaper Hong Kong
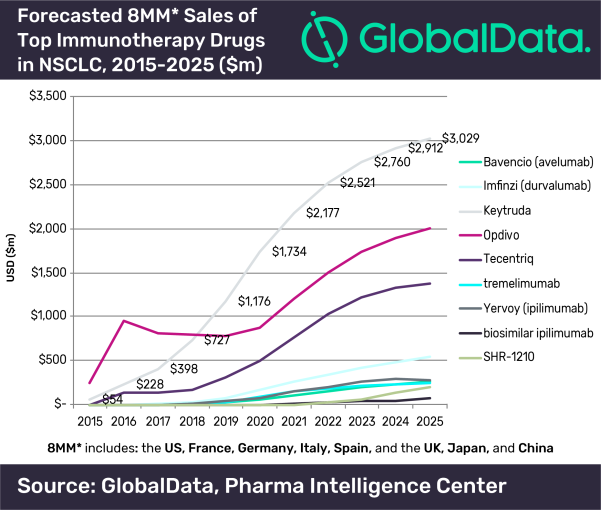
Approval of Keytruda in Europe for first-line use with chemotherapy in lung cancer strengthens the use of PD-1 inhibitors as backbone therapy, says GlobalData - GlobalData

In a first, Merck's Keytruda snags FDA nod for esophageal cancer as stomach cancer label hangs in the balance | FiercePharma

Merck's Keytruda (pembrolizumab) Receives the US FDA's Approval for Label Update to Treat Advanced Urothelial Carcinoma

Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic - Annals of Oncology

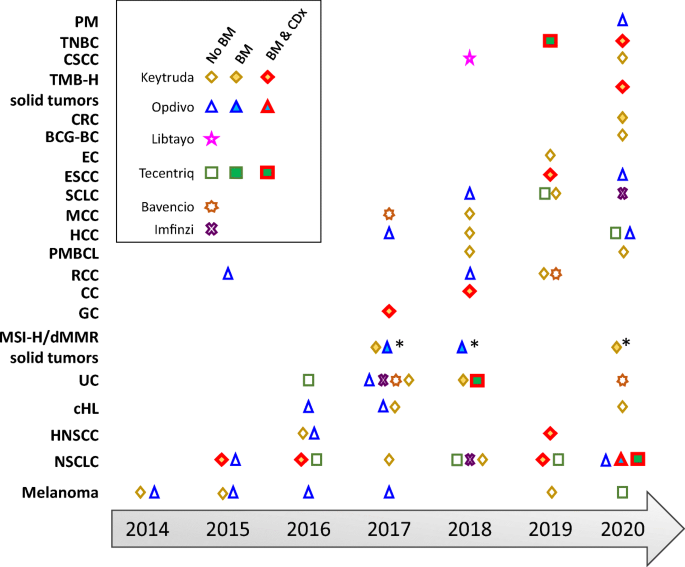
Time line of FDA approvals for the anti-PD-1 antibodies pembrolizumab... | Download Scientific Diagram